is gold dust a heterogeneous mixture
Why gold is a homogeneous mixture. Classify chunky peanut butter as an element compound heterogeneous mixture or homogeneous mixture.
Why gold is a homogeneous mixture.

. We can group mixtures further by dividing them into those that are heterogeneous and those that are homogeneous. Is a gold ring a heterogeneous mixture. Gold is neither a compound nor a mixture.
The amount of gold in the jewellery is measured in karats 24 karat would be pure gold while 18 karat is only 75 gold. Gold bars gold nuggets gold dust coins and jewelry are all made of the. A mixture is made up of two or more substances and in a heterogeneous mixture those substances are not uniformly distributed meaning that the substances that make up the mixture can be distinguished from one another upon examination.
It is not a heterogeneous mixture because a heterogeneous mixture can be physically separated. Classify oil and water as an element compound heterogeneous mixture or homogeneous mixture. There isnt a difference in the gold.
The gold in jewellery is not pure gold but is a mixture of metals. Conglomerate rock water and oil a salad trail mix of gold dust mixtures and interactive silver. Hence Jewellery gold is a heterogeneous mixture of metals.
What is a HETEROGENEOUS mixture. Hence Jewellery gold is a heterogeneous mixture of metals. Therefore it is not a mixture.
Gold for example is element number 79. There are currently 115 known elements listed on The Periodic Table of Elements. A heterogeneous mixture is a mixture of two or more chemicals in which the various components can be visually distinguished.
Gold for example is element number 79. Chalk powder and water form a heterogeneous mixture hence it is possible to separate them using physical processes. Gold bars gold nuggets gold dust coins and jewelry are all made of the same type of atoms and they cannot be broken down into anything simpler.
The only reason it is called gold dust is because gold dust is very small flakes of gold often found while gold panning. What is a COMPOUND. The pseudo-first-order removal rate of a trace gas X kIX due to the heterogeneous reaction with mineral dust depends on its average molecular speed cX the surface area concentration of mineral dust aerosol Sa and the up-take coefficient given by Eq.
Gold bars gold nuggets gold dust coins and jewelry are all made of the same type of atoms and they cannot be. One example of a mixture is airAir is a homogeneous mixture of the gaseous substances nitrogen oxygen and smaller amounts of other substancesMixtures are unlike chemical compounds because. Gold for example is element number 79.
Classify gold as an element compound heterogeneous mixture or homogeneous mixture. Conglomerate of rock water and oil A salad trail mix mix mixtures of gold dust and. It is an element that can be found in the Periodic Table of the Elements.
The substances in a mixture can be separated using physical methods such as filtration freezing and distillation. A heterogeneous mixture consists of visibly different substances or phases. An example of a heterogeneous mixture is trail mix where we can mix various components in any fraction and after mixing find heterogeneity in component distribution.
Gold for example is element number 79. Atomic Number in the Periodic Table. Is gold a homogeneous mixture.
A heterogeneous mixture is a mixture of two or more chemicals in which the various components can visually distinguish. Gold is metal that is a homogenous mixture. Why is gold a homogeneous mixture.
A mixture of silver copper pure gold and a trace of zinc gives yellow gold jewelry its rich shine. That means that it is the same all the way through and in an ideal state of purity contains nothing but gold atoms. Elements cannot be broken down any further without losing their physical chemical properties.
Gold bars gold nuggets gold dust coins and jewelry are all made of the same type of atoms and they cannot be broken down into anything simpler. Gold bars gold nuggets gold dust coins and jewelry are all made of the same type of atoms and. A mixture consists of different types of atoms or molecules that are not chemically linked.
What is a HOMOGENEOUS mixture. Of a single type of atom. A homogeneous mixture is a type of mixture in which the composition is uniform and each part of the solution has the same.
Heterogeneous reactions of mineral dust aerosol 11729 tions. Different parts are seen not uniform. Salt and pepper form a heterogeneous mixture.
Why gold is a homogeneous mixture. How to separate gold dust from sand - scuderiavalfregiait. A homogeneous mixture is a type of mixture in which the composition is uniform and each part of the solution has the same properties.
A heterogeneous mixture is a mixture of two or more chemicals where the various components can be visually distinguished. Why gold is a homogeneous mixture. Homogeneous mixtures A homogeneous mixture is a mixture of two or more chemical substances elements or compounds in which the different components cannot be visually distinguished.
Compounds Mixtures. Gold dust is a compound. How are ELEMENTS organized.
Chocolate chip cookies are a heterogeneous mixture. Answer 1 of 2. In such a case it is as close to a uniform solid solution as may be so it will be anhomogeneous mixture.
Gold is an element. Thus we can use simple. The atoms in one piece of gold are identical.
Is gold in a ring a compound. Gold for example is element number 79. The salad of gold dust mixtures and interactive silver dust.
A homogeneous mixture is a type of mixture in which the composition is uniform and every part of the solution has the same properties. Chalk powder doesnt mix with water and remains suspended as a residue in the bottom of the vessel in which the mixture in contained. While the term heterogeneous mixture sounds like it could be a complicated concept in reality it is actually quite simple.
Gold bars gold nuggets gold dust coins and jewelry are all made of the same type of atoms and they cannot be broken down into anything simpler. Explore the interactions that They cause the separation of water and oil from a mixture. Jewelry gold is a mixture because it contains more than one element type of atom and its composition varies.
Jewellery gold is a homogeneous mixture of metals.
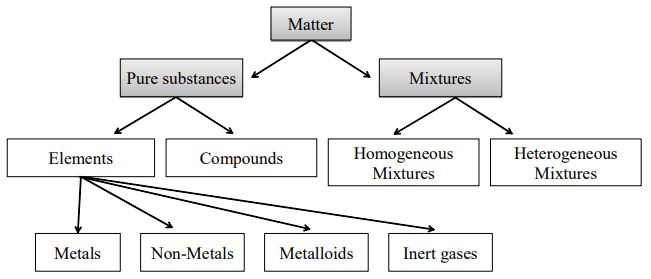
Is Matter Around Us Pure Practically Study Material

Pin By Carrie Flanagan On School Stuff Chemical Science Matter Science Chemistry Classroom

Elements Compounds Mixtures Mr Franklin S Science Lab

Our Parchment Check Fluted Plates Work Well To Add A Touch Of Simple Luxury To Any Table Setting Mackenzie Childs Mackenzie Childs Inspired Pretty Cups
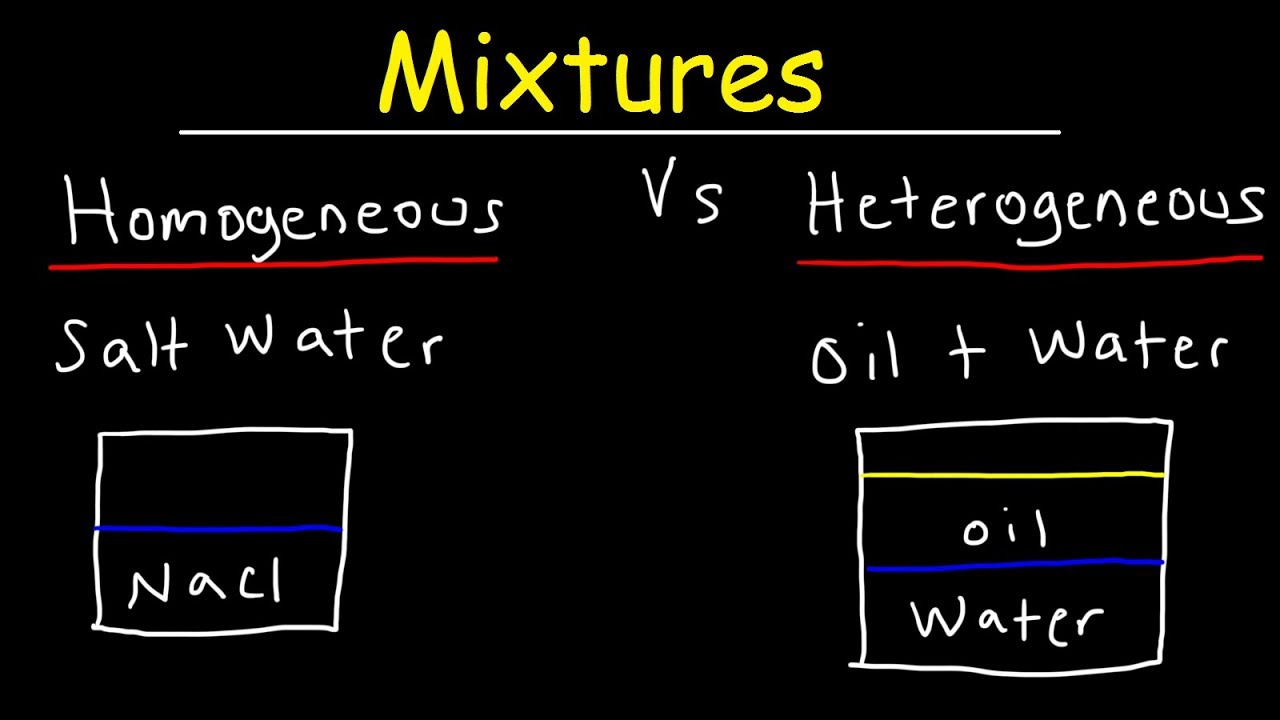
Pure Substances And Mixtures Elements Compounds Classification Of Matter Chemistry Examples Youtube

Rose Gold A Satin Ice Shimmer Color Mix Color Mixing Shimmer Satin Ice Fondant

Super Gold Luster Dust Mix Luster Dust With A Small Bowl Of Vanilla Extract The Gold Dust Cannot Be Mixed Luster Dust Dessert Decoration Gourmet Recipes

Homogeneous Mixture Definition Examples Tutors Com

Elements Compounds And Mixtures Doodle Notes Video Compounds And Mixtures Elements Compounds And Mixtures Science Notes

Elements Compounds Mixtures Mr Franklin S Science Lab

What Is A Homogeneous Mixture Definition And Examples Homogeneous Mixture Heterogeneous Mixture Compounds And Mixtures

3 5 Pure Substances And Mixtures Chemistry Libretexts

Types Of Mixtures Video Khan Academy

Pure Substances And Mixtures Elements Compounds Classification Of Matter Chemistry Examples Youtube




0 Response to "is gold dust a heterogeneous mixture"
Post a Comment